INTRODUCTION
Cancer of the oropharynx (i.e., part of the throat at the back of the mouth behind the oral cavity) is one of the most frequently diagnosed cancers worldwide, representing the seventh largest incidence burden of new cancer in men and fourteenth amongst women.1–4 Lingual squamous cell carcinoma (LSCC) accounts for approximately 3.0% of oropharyngeal carcinomas,4 typically affecting male smokers older than 45 years of age.5,6 Although LSCC is rare, global rates have been shown to increase from 0.4% to 3.3% during recent years, and there is an increasing incidence in the young female population.7
LSCC primarily confers metastatic risk to the lungs, heart, and bones, although it has demonstrated the ability to metastasize to nearly all organ systems.3 As a result, presentations of metastatic LSCC are exceptionally variable and contingent on the site of metastasis. It has also been estimated that metastatic LSCC will show cardiac involvement between 1.5% to 24.0% of all cases.8
Most cases of LSCC cardiac metastasis are detected post-mortem, although ante-mortem cases can be detected when symptomatic.9 Symptoms of cardiac metastasis are relatively non-specific, but can include fatigue, chest pain, orthopnea, and leg edema.8 To date, characterization of metastatic LSCC of the heart has been limited to case reports and case series.
Purpose of Review
The purpose of this systematic review was to examine the patient demographics, characteristics, management, treatments, complications, and outcomes associated with LSCC cardiac metastases.
METHODS
This systematic review was completed in 2020 using the Preferred Reporting Systems for Systematic Reviews and Meta-Analysis (PRISMA) guidelines.10 Preliminary searches were performed using data from PubMed, Embase, and Cochrane Library comprising studies dated through December 2019. The primary search included the keywords “tongue cancer”, “lingual cancer”, “buccal cancer”, and “metastasis”. A secondary search was done using the keywords “cardiac metastasis” and “tongue”.
The selected articles were combined to create a composite list of 1,007 studies to review. Studies that included review articles, textbooks, non-human subjects, non-English language, or unrelated topics were excluded. The inclusion criteria utilized in this systematic review are outlined in Figure 1.
Study selection and data extraction
Each article within the composite list of studies was reviewed for inclusion by two independent authors (CK, TN). The titles and abstracts were screened for information regarding metastatic LSCC presentation, diagnosis, and management. Relevant articles were further examined via full text review and a finalized list was generated for in-depth analysis (Supplemental Table 1). A total of 31 cases of LSCC cardiac metastasis within 28 studies met inclusion criteria and were comprehensively reviewed (Supplemental Table 2).
All 28 selected studies had obtained an Oxford Centre for Evidence-Based Medicine (OCEBM) evidence categorization of Level 4 given their status as either a case report or case series. Relevant patient demographics, symptoms, LSCC history, clinical findings, treatment strategies, complications, and outcomes were collected. It was assumed death had occurred within six months if patients underwent palliative care.
RESULTS
Sample Demographics and Exposure
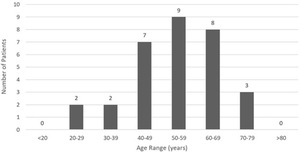
An analysis of demographic characteristics of patients in selected articles included 19 (61.3%) males and 12 (38.7%) females with a mean age of 53.6 (SD = 12.9) years ranging from 23.0 - 77.0 years. LSCC cardiac metastasis presented primarily between the ages of 40 - 69 (Figure 2). Average time from primary cancer diagnosis to cardiac metastasis identification was 2.2 (SD = 2.6) years, ranging from 0.2 - 11.0 years. Ten (32.3%) patients reported significant tobacco exposure and five (16.1%) patients admitted to alcohol use.
Presenting Symptoms and Physical Examination
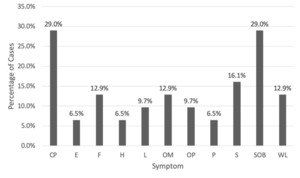
Chest pain and shortness of breath were the most common causes of initial presentation with nine (29.0%) cases, followed by five (16.1%) cases with syncope, four (12.9%) cases with weight loss, four (12.9%) cases with fever, four (12.9%) cases with oral mass, three (9.7%) cases with lymphadenopathy (i.e., enlargement of lymph nodes > or = 1), and three (9.7%) cases with oral pain. Other symptoms included two (6.5%) patients with edema, two (6.5%) patients with hemoptysis (i.e., blood mixed in sputum), and two (6.5%) patients with palpitations. A complete outline of patient symptomatology is depicted in Figure 3.
The most common physical examination findings included four (12.9%) patients with hypotension, four (12.9%) patients with fever, three (9.7%) patients with tachycardia, and three (9.7%) patients with cardiac murmur.
Cardiac Evaluation
Electrocardiogram (ECG) testing was reported in 19 (61.3%) patients, which found the most common abnormality to be ST-segment elevation in 12 (63.2%) patients (Supplemental Table 3). Other commonly reported ECG findings included arrhythmia in six (31.6%) patients, bundle branch block in four (21.1%) patients, t-wave inversion in four (21.1%) patients, and tachycardia in three (15.8%) patients. Troponin testing was reported in 31 patients with positive troponin elevations occurring in five (16.1%) cases.
Imaging Modalities and Findings
The most utilized imaging modalities were echocardiogram and computed tomography (CT) both occurring in 24 (77.4%) cases, followed by positron emission tomography (PET) in 12 (38.7%) cases, and cardiac magnetic resonance imaging (CMRI) in nine (29.0%) cases (Figure 4). On echocardiogram, the most common finding was pericardial effusion occurring in seven (29.2%) cases, followed by six (25.0%) cases with right ventricular outflow tract obstruction, three (12.5%) cases with valvular dysfunction, and two (8.3%) cases with wall motion abnormality (i.e., kinetic alterations in the cardiac wall motion taking place during the cardiac cycle) (Supplemental Table 3).
Of the patients with reported valvular dysfunction, two (66.6%) cases had impaired function of the pulmonic valve and one (33.3%) case had impaired tricuspid valve function. In the 12 patients who underwent PET scan, extra-cardiac uptake was most reported in the lung with five (41.7%) reported cases, followed by two (16.7%) cases in the bone, two (16.7%) cases in the liver, and one (8.3%) case in the muscle. The number of extra-cardiac foci ranged from 0.0 - 7.0 sites with an average of 1.8 (SD = 1.7) sites.
Location of Cardiac Metastasis
Out of 31 selected cases, 27 disclosed the number of cardiac metastases. Number of distinct cardiac metastases ranged from 1.0 - 4.0, with an average of 1.5 (SD = 0.8) metastases. Most cases presented with one individual mass in 17 (63.0%) cases, followed by two masses in 8 (29.6%) cases, and three or more masses in 2 (7.4%) cases.
The locations of metastases were distinctly specified in 30 of 31 cases. Right-sided metastases were most common with 23 (76.7%) instances, followed by 15 (50.0%) instances of left-sided metastases, and 11 (36.7%) instances of septal metastases. The most common location of metastasis was the right ventricle with 17 (56.7%) instances, followed by the left ventricle in 13 (43.3%) instances, interventricular septum in nine (30.0%) instances, and right atrium in six (20.0%) instances. This analysis also revealed eight (26.7%) patients with pericardial metastasis. Locational distinctions are further defined in Supplemental Table 2 and Supplemental Table 4.
Patient Management
Primary LSCC treatment strategies were discussed in 29 (93.5%) of 31 total cases. The most frequently reported primary treatment was surgery in 25 (86.2%) cases, followed closely by radiotherapy in 23 (79.3%) cases, and chemotherapy in 13 (44.8%) cases. The most common surgical intervention was neck dissection in eight (32.0%) cases, followed by partial glossectomy (i.e., removal of tongue tissue) in six (24.0%) cases, and hemi-mandibulectomy (i.e., removal of part of or all of the hemimandible, or one side or half of the mandible) in two (8.0%) cases.
Treatment strategies for metastatic lesions were described in 22 (71.0%) of 31 cases which included chemotherapy, surgical intervention, radiotherapy, immunotherapy, and palliative treatment. Given the poor prognosis, the most elected therapy was palliative treatment in 15 (68.2%) instances. Chemotherapy was the next most common intervention in nine (40.9%) instances, followed by surgery in four (18.2%) instances, radiotherapy in three (13.6%) instances, and immunotherapy in one (4.5%) instance.
Of those treated with chemotherapy, the most used agents were cisplatin in three (33.3%) cases and 5-fluorouracil in three (33.3%) cases. Other chemotherapeutics included bleomycin, etoposide, and methotrexate. Of note, immunotherapy with pembrolizumab was utilized in only one (4.5%) case. Individual treatments are further outlined in Supplemental Table 2.
Patient Outcomes
Twenty-two total cases (70.9%) reported outcome results, of which none showed remission of LSCC cardiac metastasis. Death was reported in 20 (90.9%) cases with two (9.1%) cases reporting palliative care and assumed death in less than six months. Of the 14 cases to report death within a known period, 9 (64.3%) reported death within one month of LSCC cardiac metastasis diagnosis.
Cause of death was listed in 16 cases which included eight (50.0%) deaths from cardiac metastasis, two (12.5%) deaths from sepsis, two (12.5%) deaths from arrhythmia/sudden cardiac death, one (6.3%) death from heart failure, one (6.3%) death from hemoptysis, one (6.3%) death from death in sleep, and one (6.3%) death from tumor embolism. Four (25.0%) cases had cardiac-related deaths, while 12 (75.0%) cases had tumor-specific or non-cardiac deaths.
DISCUSSION
Primary squamous cell carcinoma (SCC) accounts for more than 90% of cancer diagnoses within the head and neck region and is the sixth most common cancer overall by incidence.11 Classically, LSCC displays a predilection for the elderly male population,12 although it can present more aggressively in younger individuals with higher rates of metastasis and mortality.13 Recent research also suggests that young Caucasian females are demonstrating an overall increase in disease incidence.7 In this study, the majority of patients (51.6%) were males aged 40-69 years old, consistent with the typical presentation of LSCC.
In addition to age and sex, modifiable factors such as tobacco and alcohol exposure carry significant independent and synergistic risks for LSCC.14,15 Exposure to viral infections such as Epstein-Barr virus (EBV) and human papilloma virus (HPV) have also been associated with LSCC.16,17 In this systematic review, most cases failed to describe previous history of alcohol or tobacco use and no cases reported previous EBV or HPV exposure.
Cardiac metastasis of LSCC is typically diagnosed post-mortem on autopsy due to limited specific clinical manifestations.18 When diagnosed ante-mortem, patients may present with nonspecific cardiac symptoms such as chest pain, shortness of breath, and palpitations.19 In this systematic review, patients commonly demonstrated nonspecific cardiac symptoms such as chest pain, shortness of breath, syncope, and weight loss. Of these symptoms, chest pain and shortness of breath were the most prevalent (29.0% of cases), followed by syncope, then weight loss. Common physical exam findings were generally nonspecific (e.g., hypotension, fever, tachycardia, and cardiac murmur) in the selected articles.
Primary SCC originating from the head and neck with cardiac metastasis is extremely rare, as most cardiac neoplasms occur secondarily from areas such as the breast, lung, and esophagus.20 When present, cardiac metastasis most frequently occurs in the pericardium, followed by the myocardium, epicardium, endocardium, and intracavitary region.21
In this systematic review, right-sided cardiac metastasis was more common than left-sided metastasis, and ventricular involvement was more common than atrial involvement. The examined cases demonstrated a higher incidence of intracardiac tumors compared to pericardial tumors. Possible explanations for these findings relate to the hematogenous spread of squamous cell neoplasms through the coronary arteries, direct contiguous extension, and retrograde lymphatic flow.22
Since symptomatic presentations of LSCC cardiac metastasis can be highly variable, imaging studies are needed to assess the extent of cardiac involvement. First-line imaging for cardiac metastasis of LSCC begins with an echocardiogram due to its wide availability and lack of radiation.23 When there are no contraindications, contrast-enhanced CT scans are the diagnostic imaging modality of choice, as non-contrast CT scans may fail to identify small myocardial masses.24 If further imaging is indicated due to inconclusive CT results, CMRI is considered the most definitive imaging modality for evaluation of myocardial metastasis and delineation of intracardiac tumor thrombi.23
Cervical lymph node involvement is a strong prognostic factor when delineating patient outcomes for LSCC.25 Five-year survival rates for patients with oral SCC lymph node metastasis are low at 25-40%, which contrasts with a 90% survival rate for individuals without lymph node metastasis.12 Metastases to the heart further worsen this prognosis with a median survival of approximately 3.5 months without treatment.26–28 Palliative care is typically indicated for LSCC cardiac metastasis patients, as most cases present with poorly prognostic advanced cardiac SCC metastasis.26
During this review, no example of remission or significant survival was demonstrated in patients with cardiac metastasis of LSCC. Many cases resulted in cardiac-related death within 30 days of cardiac metastasis presentation and five deaths occurred upon initial admission for metastatic evaluation. These findings suggest that palliation may be the most appropriate treatment strategy for patients with LSCC cardiac metastasis given the poor prognosis with current treatment strategies.
Review Limitations
This systematic review had several limitations. First, only 31 cases of cardiac metastasis from LSCC met inclusion criteria which limits the power of this study. Second, LSCC cardiac metastasis may have been underreported in the medical literature due to missed diagnoses from lack of symptomatology or post-mortem follow-up. Third, the results of this study may not be completely representative of all documented cases of LSCC cardiac metastasis due to the omission of many non-English case reports and case series.
CONCLUSIONS
Cardiac metastasis from primary LSCC demonstrates a dangerous, uncommon presentation of malignancy. Clinical suspicion for LSCC cardiac metastasis should arise in patients with new onset chest pain and shortness of breath in the setting of prior diagnosis of LSCC. Prior tobacco and alcohol use should generate additional diagnostic speculation in these patients.
In the setting of previously known disease, advanced imaging such as echocardiogram, CT, and CMRI may prove useful for identification of disease progression. LSCC cardiac metastases typically favor the right and left ventricles, but are not exclusive to these sites. Due to the poor prognostic implications of LSCC cardiac metastasis, myocardial biopsy is unlikely to alter patient management and palliative discussions should be considered.
Conflict of Interest
None
Financial Support
None